1. Chemical bonds
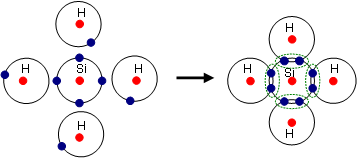
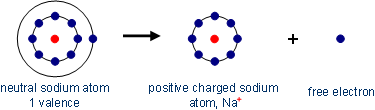
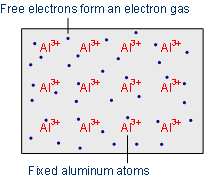
Electrons located on the outermost shell, can escape from their atoms by supplying energy (e.g. in the form of heat) and be exchanged with other atoms. Compounds of several atoms are called molecules. The reason for the bond effort is the so-called noble gas configuration - a fulfilled valence shell - which represents an energetically stable state. Substances which have reached a full outer shell, will not form bonds (a few exceptions such as xenon-fluorine compounds are possible).
There are mainly three different types of bonds, which are discussed below.