1. The atomic model
An atom is the smallest chemically not further divisible component of matter. Depending on the atom, it is composed of a certain number of electrons, protons, and neutrons. The positively charged protons and the neutrons form the nucleus, which is encircled by the electrons in certain intervals. A naturally occurring atom is electrically neutral, there are just as many positive protons as negatively charged electrons inside an atom. While the number of neutrons can vary. The simplest atom is the hydrogen atom, with only one electron, one proton, and no neutron. The next heavier atom, the noble gas helium, consists of two electrons, two protons, and two neutrons.
According to the Bohr model of the atom the electrons are assigned to so-called shells, which represent different energy levels and therefore are arranged concentrically around the nucleus. There is a maximum of seven shells, which can hold a different number of electrons, the electrons assignet to the outermost shell are known as valence electrons.
Simplified illustration of a neon atom
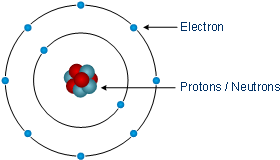
The efforts of all the atoms is to fulfill their outermost shell, with eight electrons they reach the so-called noble gas configuration (also electron octet). Elements with few outer electrons can donate electrons, elements with many outer electrons can accept additional valence electrons (see chapter chemical bonds for details).

