1. Noble gases and the octet rule
Noble gases are the elements in the eighth maingroup of the periodic table: helium, neon, argon, krypton, xenon, radon (top down).
The specificity of these elements is that they have eight electrons in their outer shell. This octet represents a very stable energetic state, which all elements would like to achieve. In the chapter chemical bonds it is explaind how the elements reach a full outer shell. Due to the stable state of the noble gas configuration, these elements will not go into reaction with other elements (a few xenon-fluorine compounds and others are known).
Noble gases
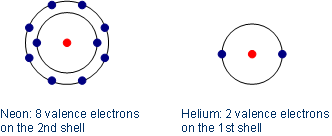
Neon consists of ten electrons: two on the first shell and eight valence electrons on the second/outer most shell. Exception: helium, which consists of only one shell, reaches the noble gas configuration already with two electrons (electron duet instead of octet).
As inert gases [lat. inert: inactive, neutral] oxygen (eg as purge gas), argon (sputtering), and others are used in the semiconductors industry.